A Closer Look at Egg White Proteins: Part 1
A Closer Look at Egg White Proteins: Part 1

Protein Theory
A good analogy for understanding egg white proteins is knitting. Think of a ball of yarn. By applying a little force, you can unravel that ball of yarn. Then, you'll put it back together by applying more force (by knitting) to create a new pattern in the form of a sweater, scarf, or gloves.
The original network of yarn in the ball is made up of the same yarn "particles" as the finished scarf or gloves. The particles have merely changed to form a different structure. When the yarn forms a different structure, it can do different things.
This is denaturing protein in a nutshell: changing the original state of the protein to form something different.
click to enlarge (desktop)
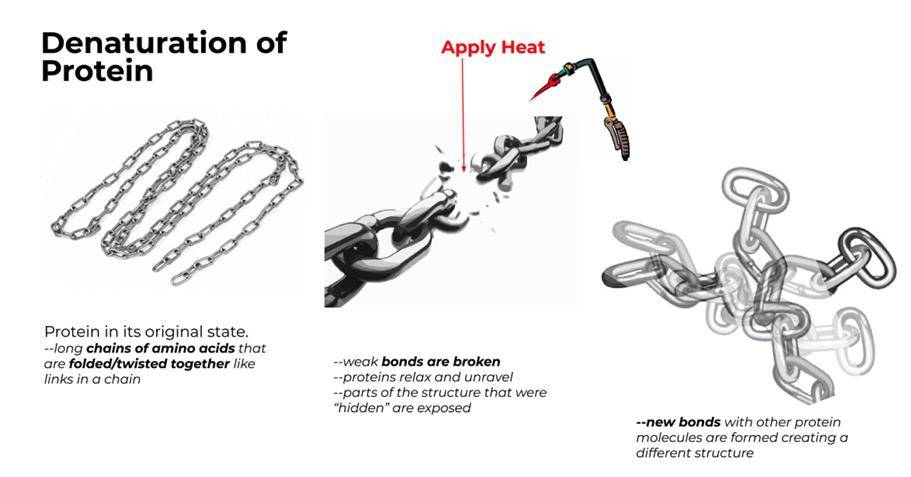
Denaturation of Protein
Proteins are created by connecting amino acids end-to-end like the links in a chain.
Chemical bonds within that chain cause proteins to fold into knot-like formations--like the ball of yarn. These formations, or structures, determine the protein's function in a recipe. Whether the proteins are tightly or loosely bound, whether the egg whites are aged or fresh, the proteins will start to open up/unfold when heat (energy) is applied.
So when you apply heat (cooking something over a bain marie, for example), the structure of the protein changes, causing it to perform differently than it would in its original state.
The process of breaking down and changing the structure of a protein is known as denaturation.

Here, the chef has added cream of tartar to the egg whites and now heats them over a water bath. Later, he will whisk the whites in a stand mixer.
Methods of Denaturing Protein
When working with whites, any one or combination of two or more manual methods of denaturing proteins may be used.
- Heat
- Example: cooking whites with sugar over a bain marie, pouring hot sugar syrup into whipping egg whites.
- Heating increases kinetic energy so the protein chains vibrate. More heat = more motion. Eventually, if enough heat is applied, it will cause the bonds that hold the proteins folded to break. The knots unfurl, and the proteins denature.
- Acid
- Example: adding cream of tartar or lemon juice to egg whites before whipping.
- Adding acid changes the pH & promotes coagulation which denatures the protein. Acids also help reduce the chances of overbeating the whites. Just don’t overdo it on the acid...it will affect taste, flavor, and, ultimately, the consistency of your product.
- Force
- Example: whipping with a whisk or mixer
- The movement and energy of rapidly moving the egg whites cause a change in their structure. Often, force is combined with heat, as when making a Swiss meringue.